Normal Alpha Olefins Resources
Chemical Reactions
Addition of Halogens
Chlorine and bromine react readily with alpha olefins to form 1,2-dihalides. Since the reaction is highly exothermic, the halide addition should be carried out slowly with cooling. Primary bromides may be formed by the free radical anti-Markownikoff addition of hydrogen bromide. Hydrogen chloride and alpha olefins will not react in a free radical anti-Markownikoff manner because the H-Cl bond strength is too strong. Secondary alkylhalides may be formed by the Markownikoff addition of hydrogen halides using weak Lewis acid catalyst.
![]()
![]()
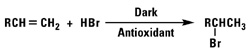
Addition of Hydrogen Bromide
Secondary or primary alkyl bromides will result from the reaction of hydrogen bromide with our normal alpha olefins by Markownikoff or anti-Markownikoff addition.
![]()
Addition of Hydrogen Chloride
Using a weak Lewis acid catalyst, hydrogen chloride reacts with normal alpha olefins to form secondary alkyl chlorides. The reaction is a typical Markownikoff addition. Hydrogen chloride and normal alpha olefins will not react in an anti-Markownikoff manner via the free radical reaction.
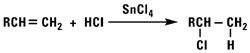
Addition of Hydrogen Sulfide
When hydrogen sulfide is added to normal alpha olefins, mercaptans and sulfides are produced. The relative yields of each can be influenced by reaction conditions and reactant concentrations. In the absence of free radicals, Markownikoff addition will occur. However, the presence of peroxides or UV light will induce anti-Markownikoff addition.
![]()
![]()
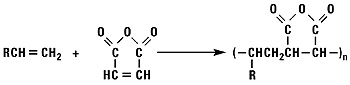
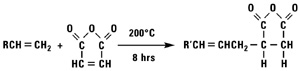
Addition of Maleic Anhydride
Normal alpha olefins and maleic anhydride react to form alkenyl succinic anhydrides (ASA).
Addition of Sodium Bisulfite
Sodium bisulfite can be directly added to our normal alpha olefins using peroxides or other free radical initiators, producing a sodium alkane sulfonate with the sulfonate group in the terminal position.
![]()
Addition of Sulfur Trioxide
Sulfur trioxide reacts with normal alpha olefins in a highly exothermic reaction via a 2 + 2 cyclo addition mechanism to produce a beta sultone as the initial product. The sultone is unstable and rearranges into a mixture of alkene sulfonic acids plus gamma and delta sultones.
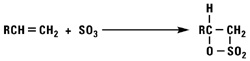
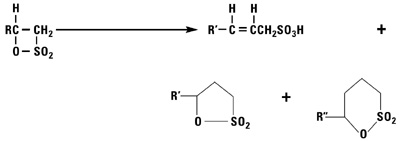
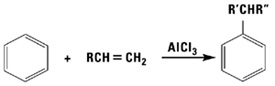
Alkylation Reactions
With acid catalysis, alpha olefins readily alkylate aromatic compounds such as benzene, phenol, xylene and toluene. The products are mixtures of linear alkyl aromatics, ideal for conversion to detergents via sulfonation or ethoxylation.
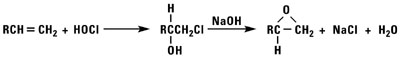
Epoxidation
Normal alpha olefins can be converted into 1,2 epoxides by the addition of hypochlorous acid followed by treatment with base, treatment with peracids, or by using t-butylhydroperoxide and a molybdenum catalyst.
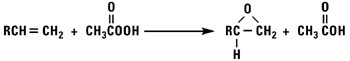
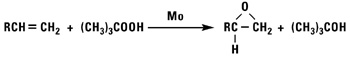
Isomerized Products
Alpha olefins can be isomerized to produce an array of mixtures of linear internal olefins and even-branched olefins.
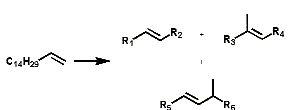
Koch Reaction
The Koch reaction produces a mixture of branched carboxylic acids from normal alpha olefins. The reaction proceeds in the absence of a solvent and is applicable to C6 to C24 normal alpha olefins.
![]()
Oligomerization and Dimerization
Normal alpha olefins can be treated with Lewis acids to produce dimers, trimers, tetramers and related compounds. The resultant products are highly branched and consequently have much lower pour points than straight-chained hydrocarbons of the same molecular weight. Dimers with a terminal double bond and an alkyl group on the second carbon of the double bond can also be produced by using an aluminum alkyl catalyst.
![]()
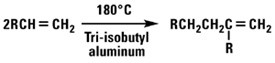
Oxo Chemistry
Our normal alpha olefins will react with carbon monoxide and a variety of nucleophiles to produce aldehydes, alcohols, esters or acid chlorides.
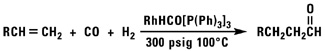
![]()
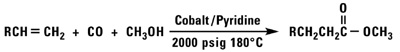

Polymerization
Ethylene and normal alpha olefins can be copolymerized by conventional catalyst systems used to produce high-density and linear low-density polyethylene. Normal alpha olefin products can also be homopolymerized with the same catalysts, producing 1-polybutene, for example.
Our normal alpha olefins can also be copolymerized with maleic anhydride or maleate esters via free radical initiators to produce alternating 1:1 copolymers.
![]()
![]()